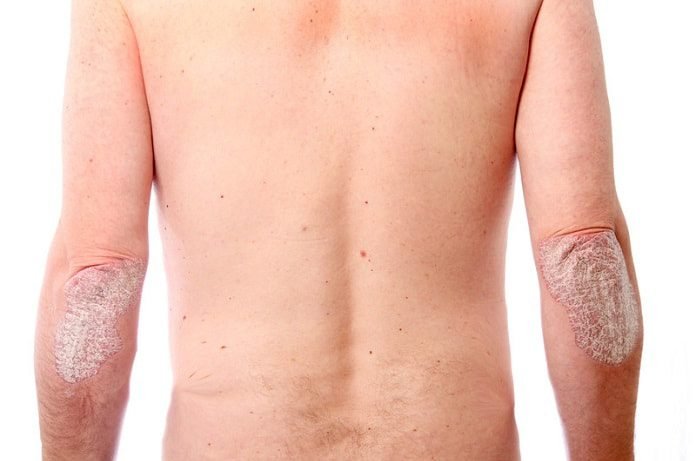
A recent randomized phase II clinical trial demonstrates that risankizumab treats psoriasis, a chronic immune-mediated inflammatory skin disease, more effectively than ustekinumab.
Psoriasis, a chronic immune-mediated inflammatory skin disease, affects 2% of adults and is associated with a poor quality of life, obesity, hypertension, diabetes, hypercholesterolemia, and metabolic syndrome. Researchers suggests that interleukin-23 (IL-23), composed of a p19 and p40 subunit, plays a significant role in the disease by inducing and maintaining inflammatory cells. Current strategies include monoclonal antibodies aimed at the different subunits of interleukin-23, including ustekinumab and risankizumab. Ustekinumab targets the p40 subunit, which is also found in IL-12, and thus acts against both IL-23 and IL-12. In contrast, risankizumab only targets the p19 subunit and selectively inhibits IL-23 activity. Clinical studies have shown that both drugs are safe, well-tolerated, and effective in treating psoriasis patients.
A recent randomized phase II clinical trial compared the efficacy, onset, and duration of clinical response between the two drugs in patients with moderate-to-severe psoriasis. Patients were randomly assigned to receive either a single 18-mg dose of risankizumab at week 0, a 90-mg or 180-mg dose of risankizumab at week 0, 4, and 16, or a dose of ustekinumab at week 0, 4, and 16. Patients were subsequently followed for 32 weeks after the final injection (total trial period of 48 weeks). The primary endpoint was a 90% or greater reduction from baseline in the PASI or Psoriasis Area Severity Index, which is an evaluation of erythema (redness), scaling, and percentage of body-surface area affected. In addition, the authors investigated safety end points including severe and moderate adverse events.
At the end of the study, a 90% or greater reduction in PASI was observed in 73% of patients in the 90-mg risankizumab group and 80% of patients in the 180-mg risankizumab group, compared with only 40% of patients who received ustekinumab. This indicates that a 90 and 180-mg dose of risankizumab is more effective in treating psoriasis than ustekinumab. The authors also found that the onset of risankizumab was earlier than ustekinumab, and the benefits were sustained for longer. Also, patient reports and skin biopsies further suggest that risankizumab was more effective in treating psoriasis and its associated morbidities than ustekinumab.
In conclusion, the randomized phase II clinical trial demonstrates that risankizumab is superior to ustekinumab in treating psoriasis and its associated morbidities. The onset and duration of beneficial effects are more profound and longer with risankizumab treatment. Although two patients developed basal-cell carcinoma and one had an adverse major cardiac event with risankizumab; the study had a small sample size and short duration, making it difficult to assess safety profiles. Nonetheless, the study suggests that selective blockade of IL-23, via p19 subunit inhibition, is more effective in treating psoriasis than inhibition of both IL-23 and IL-12. Future studies are required to confirm these results, and to better assess the safety profile of risankizumab.
Written By: Haisam Shah, BSc